Ingersoll Rand TS5 60-Gallon Vertical Air Compressor - ingersoll rand ts5
Nova has achieved ISO 13485 Quality Management System certification—quality system standards recognized by more than 160 countries. A natural evolution of Nova’s world-class quality philosophy and ISO 13485 Quality Management System certification requires that a company develop, document, and effectively implement comprehensive quality systems from research and development to manufacturing, delivery, and customer support.
Nova appoints a dedicated development and manufacturing team to your project. Manufacturing is done through a “focused factory” concept where dedicated staff, space, and equipment are permanently assigned to your project.
TGR’s journey of making ever-better motorsports-bred cars is without end. TGR intends to continue competing in races using the GR Yaris and evolving it with input from various drivers.

We’re known by the company we keep. Nova’s clients include some of the world’s best-known healthcare companies, as well as some of the most innovative newcomers.
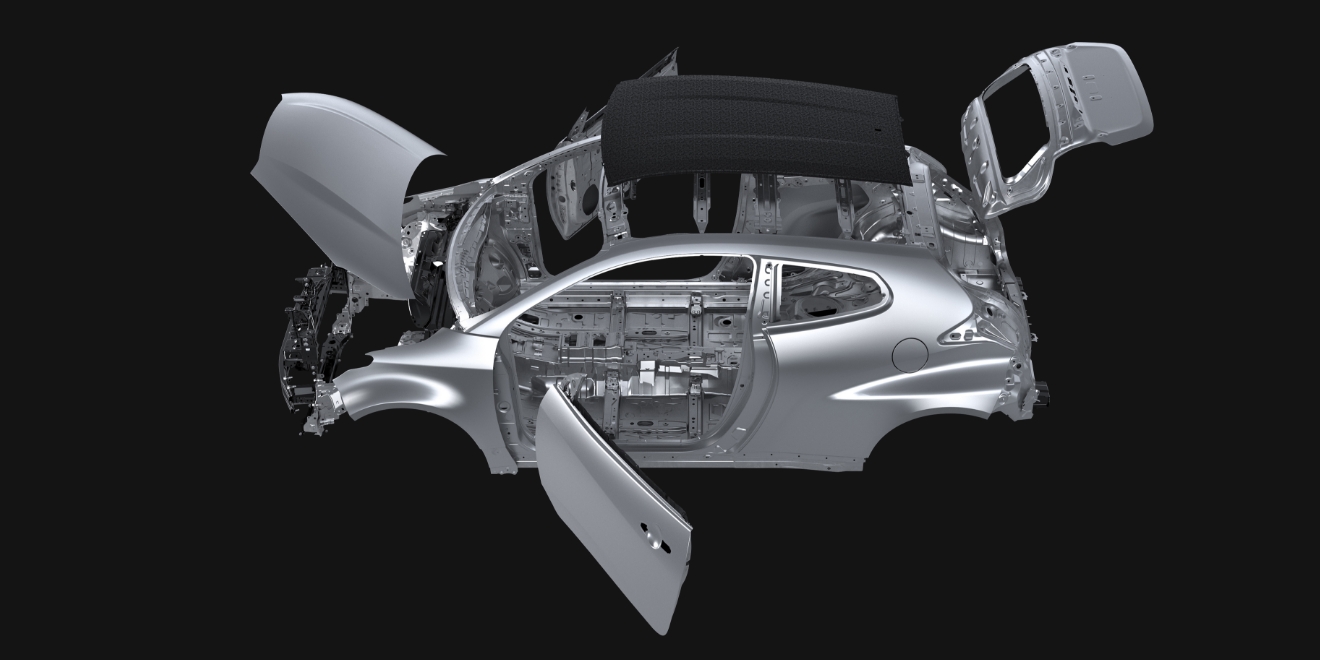
TOYOTA GAZOO Racing (TGR) announced today that sales of its evolved GR Yaris are to start on April 8 through Toyota vehicle dealers across Japan.
With a goal to improve the quality of each product and reduce its manufacturing cost, Nova provides cost reduction and manufacturing engineering support to each client on an ongoing basis at no addition charge.
For more product information, click here or contact Nova Biomedical at: Nova Biomedical / 200 Prospect Street / Waltham, MA 02454 / 781-894-0800 For Nova Biomedical Sales inquiries, reach us at 1-800-458-5813. For Technical Support, reach us at 1-800-545-NOVA (6682). To place an order, call 1-800-822-0911 or email novaorders@novabio.com
Nova is an FDA-registered manufacturer of medical products. All design processes and manufacturing procedures comply with FDA Quality System Regulations.
Nova’s clients include some of the world’s best-known healthcare companies, as well as some of the most innovative newcomers.

Nova has also achieved MDSAP certification which confirms Nova Biomedical complies with all relevant Canadian, Japanese, Brazilian, Australian, and United States regulatory requirements for medical devices.
Nova uses Manufacturing Engineering as the key constant in all projects. Manufacturing Engineering is assigned to the product development project on day one and stays with the project for the life of the product. There is no handoff from development to manufacturing. There is no redesign for manufacturing effectiveness. There is a seamless transition that is managed by Manufacturing Engineering, which ensures that the product is reliable and cost-effective in manufacturing design.
Among the capabilities that separate Nova from other manufacturers is our ability to accommodate a broad range of projects from simple to complex and low to high volume, including reagents, instrumentation, and disposables.
Also, based on Morizo’s desire to provide the fun of driving to as many people as possible and to expand the scope of motorsports, TGR has developed the 8-speed GAZOO Racing Direct Automatic Transmission to enable the utmost enjoyment of a 1.6-liter inline 3-cylinder turbo engine mated to a 4WD drivetrain, which forms the essence of the GR Yaris. The result is a car that is designed so that a wide range of drivers can enjoy sporty driving and competing in motorsports to the fullest.
As a symbol of TGR car-making, the GR Yaris was born through making ever-better motorsports-bred cars. TGR has continued to compete in various motorsports*1 using the GR Yaris since the model’s launch in September 2020. That is because problems that occur in the extreme environments of rallies and other races provide opportunities to evolve the GR Yaris into an ever-better car. Under the slogan of “Thanks for breaking it” directed at the drivers who pushed the vehicle to its limits, TGR honed the GR Yaris through constant enhancements, thoroughly pursuing the cause of problems by analyzing driving data, reviewing steering feel, and examining what kind of scratches and dirt were on broken parts.
For more product information, click here or contact Nova Biomedical at: Nova Biomedical / 200 Prospect Street / Waltham, MA 02454 / 781-894-0800 For Nova Biomedical Sales inquiries, reach us at 1-800-458-5813. For Technical Support, reach us at 1-800-545-NOVA (6682). To place an order, call 1-800-822-0911 or email novaorders@novabio.com
This process flow diagram illustrates Nova’s proven methodology for development and manufacturing of more than 100 products over the past 35 years. Nova has secured more than 100 FDA 510(k) clearances for our products and clients.
Over the past 40+ years, we have built a strong reputation based on leadership in diagnostic technology, engineering excellence, cost-effective manufacturing, and integrity.
In contrast to many contract manufacturers, Nova does not “time share” its production resources. Instead, Nova dedicates manufacturing personnel as well as space and inventory resources to each individual project.
Nova’s U.S. service organization consists of more than 40 field engineers. We have direct service organizations in Brazil, Canada, France, Germany, Japan, Italy, Spain, Switzerland, and the United Kingdom.
Nova offers component, sub-system, and complete product prototyping via a state-of-the art, parametric solids model CAD/CAM system, a prototype shop, and 3-D printers.
Our standard quality procedures include organization and training, incoming QA, in-process inspection, full system functional testing, and statistical analysis. Importantly, we also form a Product Line Quality Committee (PLQC) for each product that is comprised of Nova and client participants. The PLQC is an oversight group that proactively addresses a wide range of quality and operational issues.
Nova Biomedical is a world leader in the development and manufacturing of advanced technology blood testing analyzers and diagnostic products. As the largest privately held in vitro diagnostic company in the U.S., we employ over 1,200 people, including over 125 scientists and engineers, and have sales and service subsidiaries in nine countries and distributors in over 92 other countries. Nova also has 420,000 square feet of development and manufacturing space in facilities in Waltham and Billerica, Massachusetts; and Taipei, Taiwan.
During a development or manufacturing program, our innovative quality control program establishes cross-functional quality teams, including members of our clients’ staff, to proactively address quality and operational improvements throughout the duration of each project. This intensive philosophy results in a level of quality that consistently surpasses our clients’ expectations.
This focused factory approach clearly identifies responsibility and promotes the most effective operation throughout the manufacturing process including: manufacturing management, technical support, production control and planning, quality control, production supervision, and inventory warehouse.
Nova’s engineering and product development expertise begins with world-class experience in both optical and electrochemical diagnostic technologies for blood chemistry and immunoassay applications. To this we add strong project management with computer-based electronic and electromechanical systems experience. We place particular emphasis on designing for reliability, value, and manufacturing readiness. Key mechanical skills include optics, precision fluid handling, heat transfer mechanisms, plastics, packaging, and industrial design. Electronic skills include both analog and digital circuitry, embedded microprocessors, ESD, and power supplies.
Our unique “open-book” pricing model identifies costs for materials, overhead, labor, and profit associated with the project. This full disclosure policy keeps each client fully appraised of the financial status of a project every step of the way.
Nova’s software engineers are proficient in real-time control, multi-tasking operation, and custom-embedded systems software. All products are designed for worldwide sales and marketing, with appropriate regulatory certifications.
Our production capabilities range from high volume, low cost products to low volume, high cost products. We have structured our “focused factories” to accommodate this range of product mix.




 Neil
Neil 
 Neil
Neil